
Like all other objects
molecules have a resonant frequency. This means that they vibrate wildly when a certain
input frequency is applied to them in just the same way that a child's swing will build up large
oscillations if it is pushed at just the right rate.
Of course, molecules are complicated
things containing two or more atoms and so working out the resonant frequency is tricky.
However it is possible to calculate the resonant frequency of diatomic (two atom) molecules
and some results are shown below.
Hydrogen chloride 8.66x1013 Hz
Carbon monoxide 6.42x1013 Hz
Nitrous oxide 5.63x1013 Hz
These frequencies lie in the near infra red part of
the spectrum, nowhere near the microwave area. The theory behind this calculation is really
difficult but of you are interested you can look up theory of how these frequencies worked out
in university text books.
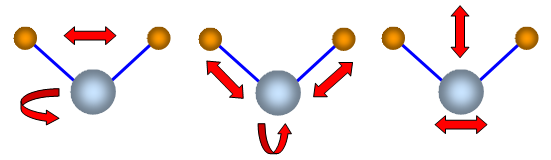
Some vibrations of a three atom molecules are shown in
the diagram. They are not to scale and are only meant to represent possible states of
vibration.
When microwaves pass through water the water molecules absorb some
of the microwave energy and as a result they twist and turn, writhing around, as the radiation
passes by. However after the microwaves have gone the molecules stop moving again,
remitting the energy as more microwaves. In free water molecules this does not result in a
heating.
In a liquid things are rather different. The water molecules are close to
reach other and so there is "friction" between them. It is the rubbing of one molecule against
another as in liquid water that allows the energy to be retained and prevents it being
reemitted as microwaves. The "friction" between the writhing water molecules and other
molecules in a solid also heats up the solid.
Microwave ovens operate at a
frequency of 2.45 GHz (2.45x109 Hz) and this is NOT the resonant frequency of a water
molecule. This frequency is much lower than the diatomic molecule resonant frequencies
mentioned earlier. If 2.45 GHz were the resonant frequency of water molecules the
microwaves would all be absorbed in the surface layer of a substance (liquid water or food)
and so the interior of the food would not get cooked at all.
The 2.45 GHz is a kind of
useful average frequency. If the frequency was much higher then the waves would penetrate
less well, lower frequencies would penetrate better but are absorbed only weakly and so
once again the food would not absorb enough energy to cook well.
Standing waves
set up within the oven. A standing wave is formed whenever two waves travelling in opposite
directions meet in a "restricted area". This restricted area could be a metal box (as in a
microwave oven) or a stretched string as in a violin.
Microwave ovens cook
unevenly because a pattern of standing waves forms inside the oven chamber, and the
pattern creates an array of hotspots throughout the oven's volume. An operating frequency of
2.45 GHz will produce a wavelength of around 12.25 cm, and the regions of maximum
intensity (hotspots) will be at half-wave points, or every 6.125 cm, but in a complex 3D
pattern.
This standing wave pattern explains why microwave ovens only work
effectively if the food is rotated through the standing waves and why some ovens actually
move the pattern by rotating the transmitter.
Microwave
ovens have a special cycle to defrost food. This is because while water absorbs the
microwaves strongly ice does not. Therefore as the ice melts the water formed gets very hot
quickly and so you can have ice and very hot water in the same portion of food. Therefore in
the defrost cycle the microwave power is switched on and off so that there is time for the
heat to spread out from the melted water.
For further details it is worth looking
at a really good site with a moving
applet: